Obtain accurate and reliable full length plasmid sequencing with our Next Generation Sequencing service.
Cell and Gene Therapy
Plasmid DNA manufacturing using an unique process for Research, HQ and GMP grades
Purity
High purity plasmids with very low endotoxin level (< 2EU/mg)
Robust
Unique scalable proprietary process allowing plasmid production from 50mg to 20g in Research, HQ & GMP grades
Time-Saving
Between 4 and 7 weeks for Research Grade and 3 months for GMP grade
Flexible
Manufacturing process compatible with all types of plasmids (from 2 to more than 20 kb, plasmids for LV, AAV, mRNA)
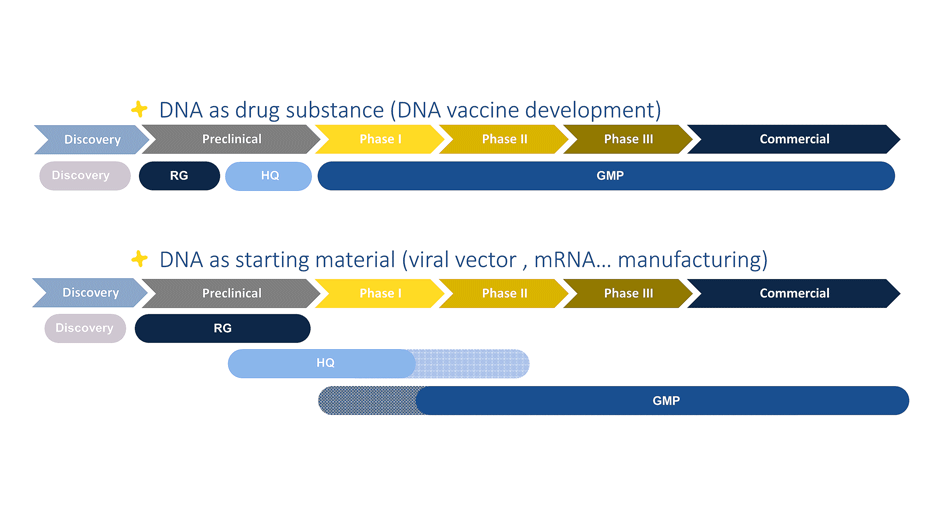
Fig :Polyplus proposes different quality grades based on the applications (drug substance vs critical starting materiall)
Polyplus offers a comprehensive range of plasmid services including DNA sequence design, optimization and plasmid engineering, cloning, transformation in our proprietary and IP free microbial E. coli, fermentation, patented soft DNA extraction process, single chromatography step and analytical testing.
Polyplus has developed an unique robust and scalable plasmid manufacturing process ensuring the highest plasmid purity possible with extremely low endotoxin levels (< 2EU/mg), whatever the scale and the quality grade, being research, HQ or GMP grade.
Our facility is equipped with state-of-the-art technology (stainless steel fermenters Sartorius Biostat® C+) and is operated by a team of experienced scientists and technicians for fast fermentation development and optimization.

These FAQs are organized by application to guide you to find the best answer possible.

You have access to all the documents related to the transfection reagent.

Search for publications in our Transfection Database with Polyplus transfection reagents

This lexicon will help you to understand the different terms related to Polyplus-transfection®.