Polyplus offers a comprehensive range of plasmid manufacturing services from DNA plasmid design to plasmid DNA manufacturing and analytical testing.

Accelerating the development and successful approval of lentiviral vectors (LVVs) for advanced therapy applications requires access to optimized and aligned plasmid and LVV manufacturing platforms. A close collaboration between VIVEbiotech, a CDMO focused on LVV development and manufacturing, and Xpress Biologics, a provider of plasmid development and manufacturing outsourcing services, addressed these critical needs. This is part of a series of projects Polyplus and Sartorius companies are working on to see how increasing quality or matching GMP-grade materials and equipment early in processes lowers time and cost.
 |
Philippe Ledent
Chief Scientific Director, Xpress Biologics (now part of Polyplus and Sartorius) |
 |
Rakel Lopez de Maturana
Qualified Person and Regulatory Affairs Director, VIVEbiotech |
Development of gene and gene-modified cell therapies continues at a rapid pace, with most candidates in clinical trials reliant on viral vectors for delivery of the relevant genetic material. One of the most common viral vectors in use today is lentivirus (LV) being used mainly for cancer and rare diseases and with an increased use for in vivo applications.
LVVs are single-stranded RNA-based retroviruses typically derived from human immunodeficiency virus (HIV).1 They have large packaging capacity (up to 5 to 8 or even 10kb), leading to stable gene expression.2,3 Vesicular stomatitis virus protein G (VSV‐G) pseudotyped LVVs can, in addition, efficiently transduce any cells (dividing and nondividing) of almost any type, including primary cells, immortalized cell lines and hematopoietic stem cells, and more pseudotypes are nowadays being identified so that more specific cell targeting is reached3.
The complexity of LVV manufacturing creates challenges with respect to meeting growing demand. It is estimated that the market for LVVs will expand at a CAGR of 18.5% from a value of $292 million in 20234 to $829 million by 20305 Cost-efficient, robust, scalable processes will be essential.
Simultaneously achieving high productivity and high product quality requires access to high-quality raw materials and well-understood manufacturing processes for both plasmids and the LVVs themselves.
As a critical raw material, plasmid DNA must meet global regulatory requirements for the European Medicines Agency (EMA) the US Food and Drug Administration (FDA) and other regulatory bodies. It must also be of suitable quality for different development phases and be available in a timely manner. In addition, a platform approach to plasmid manufacturing is needed that is sufficiently flexible to accommodate tailoring (such as modification of analytical assays) to meet the specific requirements of different individual projects.
The partnership established between plasmid manufacturer Xpress Biologics (part of Sartorius company, Polyplus) and LVV vector development and manufacturing specialist VIVEbiotech provides an optimum solution for drug developers seeking high-quality LVVs with streamlined production timelines.
VIVEbiotech has experience with production of both second- & third-generation and for the generation of LVVs using different cell types and involving a wide range of therapeutic transgenes (genes of interest or GOIs), as well as different pseudotypes. Most of the projects that VIVEbiotech is managing involve the use of third-generation LVVs, which require one transfer plasmid encoding the GOI and three helper plasmids.
The transfer plasmid comprises a promotor and the GOI flanked by long terminal repeats (LTRs). The LTRs are self-inactivating and thus the proviral DNA contains no LTR-derived enhancer or promoter elements, reducing the probability of proviral-mediated impacts on nearby genes.
Two of the three helper plasmids are packaging plasmids, one encoding GAG and POL (CMV:GAG:POL:RRE) and the other encoding REV (CMV:REV). The third helper plasmid is an envelope plasmid (CMV:VSV-G:PolyA). It should be noted that while CMV is the typical promoter, others are also employed. In addition, VIVEbiotech has worked with other pseudotypes besides VSV-G.
Transfection of the four plasmids is performed in HEK293T cells under adherent conditions in fixed-bed bioreactors using PEIpro® transfection reagent to generate LV particles. As with any transfection reaction, the longer the transfer plasmid, the more challenging it is to achieve high productivity. There is, however, room for improvement of plasmid design through promoter modification, deletion of unnecessary elements and incorporation of components from various other viruses.
Opportunities for optimization are identified based on prior experience and existing knowledge of plasmid design and performance combined with in silico assessments always considering regulatory compliance, productivity, and any specific project requirements.
More information is obtained by performing a small-scale production to generate the LVVs. Recommendations for optimization are then made by VIVEbiotech’s Innovation Department with the goal of ensuring regulatory compliance, increasing titers, and providing more cost-effective processes, ultimately raising the probability of success. If changes are made, another small-scale run is performed to confirm their impact on performance.
If necessary, VIVEbiotech also supports clients with optimization of the transient transfection process, including investigation of various process parameters, such as plasmid ratios, plasmid-to-transfection reagent ratios, among others.
Once the optimal plasmid design has been established, VIVEbiotech then subcontracts the manufacturing of the plasmids to Xpress Biologics, which is qualified as an approved plasmid supplier for the manufacture of the specific LVV product. VIVEbiotech also prepares/produces and ships any necessary starting materials to Xpress Biologics and prepares required CMD documentation for the plasmids as critical starting materials. The progress of the production process at Xpress Biologics is monitored closely by VIVEbiotech, as are all assay results (including stability tests) and product release. The plasmids are shipped to VIVEbiotech, where they are stored under controlled conditions.
The Xpress Biologics team is focused on generating plasmid products with the highest possible content of the supercoiled form (SC at least >90%) that are free of endotoxins, host germline DNA and mRNA, and host-cell proteins (HCPs).
The platform production process involves E. coli fermentation followed by centrifugation to generate a cell paste, which is lysed using a proprietary soft lysis process and then clarified. Ultrafiltration of the lysate removes impurities including pigments, HCPs, and small mRNAs, making it possible to achieve the desired purity with only a single anion exchange (AEX) chromatography step, allowing a SC enrichment and endotoxin removal to very low levels. A second ultrafiltration step is then performed to provide the plasmid in its final buffer solution.
Fermentation conditions depend on the type of plasmid. Those that contain LTR sequences and temperature-sensitive origins of replication (ORIs) require a two-step process in which a certain level of biomass is achieved under fed-batch conditions and then various process parameters (e.g., temperature, feeding rate) are adjusted to induce plasmid replication while avoiding recombination events. Simpler plasmids can be produced in one step without any adjustment of process conditions. In all cases, no limitation of oxygen is observed and there is no cell lysis and nuclease production, leading to generation of high-integrity plasmids. Using this platform process, 350 to 815 mg/L of plasmids can be produced depending on the nature of the plasmid.
Soft lysis of the cell paste takes place in three steps. First, to solubilize the cell membranes and extract the plasmids, the cell paste is mixed in a bioreactor with a solution containing sodium hydroxide and a detergent for a defined period of time. This step also separates the strands of both plasmid DNA and germline DNA.
|
|
LVV Services at Any ScaleVIVEbiotech is one of the only contract development and manufacturing organizations fully specialized in lentiviral vector development and manufacturing for both ex vivo and in vivo administration. The company produces LVVs for all development phases, including R&D grade batches, engineering batches and GMP-compliant batches up to commercial scales, with starting and raw materials, including plasmids, adapted to the phase of each project (Research, High Quality and GMP grades). Currently, VIVEbiotech is working with more than 45 customers globally using a platform process that is compliant with both EMA and FDA regulations. Customers can rely on timely slot availability and flexibility increasing capacity as their projects advance from clinical development to commercialization. VIVEbiotech continues to invest in innovation to further improve their highly optimized manufacturing platform to support the manufacture of high-quality LVVs in a cost-effective and reproducible manner. |
Next, a salt solution is added to neutralize the mixture, which promotes precipitation of the cell membrane and initiates realignment of the DNA strands. Notably, the rate of recombination of the plasmid DNA is rapid, while that for the germline DNA is much slower, allowing selective precipitation of the single-stranded germline DNA via addition of another salt solution.
Analysis of the plasmid solution at this point shows a large peak for the supercoiled form, a very small peak corresponding to the open circle form and a small massive of peaks corresponding to small-sized RNA species. Following tangential-flow filtration (TFF) to concentrate the lysate solution, an AEX chromatography step results in further SC form enrichment based on the different charge densities of the SC and OC forms (OC elutes first). A typical result in enrichment brings the SC form to above 97%. The AEX step also dramatically reduces the endotoxin level from approximately 10,000 EU/mg following ultrafiltration to approximately 1 EU/mg. Such a low level of endotoxin in plasmids is essential for realizing efficient transient transfection.
It is important to emphasize that the same process for plasmid production is employed from R&D (5 L) to GMP (50 L) manufacturing, and therefore plasmids of the same quality are obtained regardless the scale. Plasmids are offered at Research, High Quality (HQ) or GMP grade with increasing levels of quality control and monitoring, and number and variety of assays (all of which, except sequencing, sterility and mycoplasma are in-house). HQ material can be used for preclinical and early clinical testing, while GMP material is required for Phase 3 trials and commercial manufacturing. If clients desire additional testing for R&D-grade material, there is flexibility for Xpress Biologics to perform additional analyses.
This relationship has produced 20 different supercoiled plasmids at all three grades, including plasmids with sizes ranging from 4.3 to 11.4 kb. Selected specifications that have consistently been met include a SC content >90%, residual host DNA (by qPCR) and residual host RNA (by HPLC) content <2.5%, residual HCP (by microBCA) content <1.0%, and endotoxin levels (by LAL) <20 EU/mg.
LVV manufacturing at VIVEbiotech matches the specific needs of each client depending on the development phase, the dosing and other specific needs of each project both for ex vivo and in vivo use, having worked so far for 45 international clients in US, Europe, Asia and Australia. The fixed-bed bioreactors employed have surface areas ranging from 2.4 to 200 square meters.
Manufacturing Plasmids to Streamline ProcessesBelgium-based Xpress Biologics offers plasmid/sequence engineering and molecular biology services to the biopharma industry, as well as the production of tailor-made plasmids in Research, High Quality and GMP grades at scales ranging from 50 mg to 40 g (capacity expansion beyond 40 g operational in 2025). Advanced quality controls including whole plasmid sequencing by NGS ensure the highest quality products, whether plasmids are intended for use as drug substances such as for DNA vaccines or critical raw materials for production of viral vectors and mRNA. In the first two cases, plasmids are manufactured with a very high content of the supercoiled form. For mRNA, the plasmids are linearized. The proprietary, optimized plasmid manufacturing platform process is efficient and scalable with short lead times and fast delivery between 4 and 14 weeks depending on the material grade. These timelines are possible due to a unique, proprietary lysis/extraction process that reduces further purification needs to just one chromatography step, saving time and resources. While one E coli cell is predominantly employed (90% of projects), Xpress Biologics has access to two additional strains, one inhouse cell line adapted to different temperature shifts and another licensed strain with very low recombinase activity for plasmids exhibiting cellular toxicity. This broad capability has made it possible for the company to produce over 100 different plasmids, including those with very low (1) and very high (~500) copy numbers, vectors ranging in size from 1.8 to > 20 kb, and those with both conventional and more specific vector backbones, such as large and repeated sequences, hairpin sequences (e.g., LTR/ITR vectors), and pol(A) tails. |
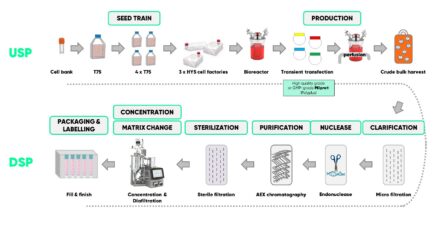
Platform LVV production begins with 2D expansion of packaging cells. The bioreactor is inoculated, the cells further expanded to an appropriate number and transient transfection performed using the appropriate grade of PEIpro (HQ or GMP). After several days of perfusion to collect the culture media, the crude bulk harvest is subjected to downstream processing, which includes clarification via microfiltration, endonuclease treatment, AEX chromatography, sterile filtration, concentration/diafiltration via TFF, and fill/finish.
The generated LVV vectors are characterized for identity, efficacy, and safety. Full sequencing from ITR to ITR is performed. Process- and product-related impurities are confirmed to be reduced to acceptable levels. Both the transduction capacity and transgene expression are measured. Comprehensive evaluation is achieved using many analytical tests developed inhouse by VIVEbiotech, including assays for residual plasmid sequences (specific real-time qPCR analyses for PSI, REV, VSV-G, TAT, KANR, GAG), total residual DNA (real-time PCR), DNA size (HPLC) and endotoxins and other impurities present in the plasmid raw materials.
Due to extensive optimization of the LVV manufacturing process, levels of residual impurities are very low and in some cases below the limit of quantitation of the relevant method.
The approaches to both plasmid and LVV manufacturing leveraged at Xpress Biologics and VIVEbiotech are similar. The same manufacturing platform is used in both companies for plasmid and LVV manufacturing, respectively, and same specifications are set regardless of the target product quality (HQ or GMP), with flexible analytical characterization that can be adapted to specific project needs. Even for projects that require plasmid optimization, GMP-grade LVVs can be produced within 6 months and released approximately 3 months later.
References