Explore our easy plasmid services starting at just $249. Our fully sequenced plasmids, ready for your research, include a miniprep. Reduce project cos...

Protein Production
Process development to manufacturing of proteins at Research, Hq and GMP grade
End to end integrated service
Fully optimized technological platforms for process development to manufacturing of recombinant protein or antibody fragments.
Compliance for preclinical and clinical use
Manufacturing capabilities at research, HQ and GMP grade to meet regulatory compliance for therapeutics, vaccines and diagnostics.
Scalability
Manufacturing capacityto produce protein quantities ranging from 100 mg to 40 g.
IP and license free
All services provided by Xpress Biologics are free of charge in terms of license or royalty fees.
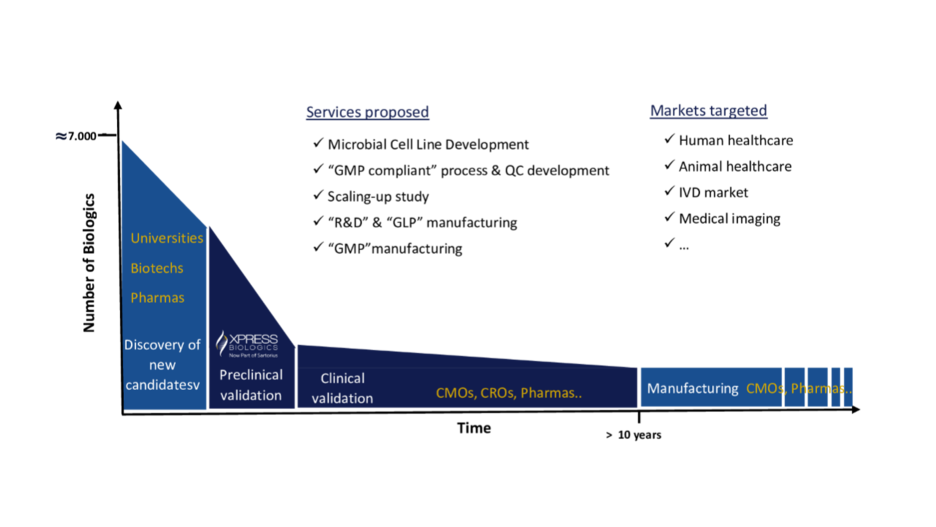
We offer an end-to-end integrated protein manufacturing service thanks to our CDMO Xpress Biologics that have since 2014 continuously grown their capabilities to meet current demands to produce recombinant proteins and antibody fragments for preclinical and clinical use, specific to human and veterinary therapeutics, vaccines and diagnostics markets. Both, the production scale from 100 mg up to 40 g and the quality of the biologics from Research to HQ (High quality/ GLP) and GMP grade proposed by Xpress Biologics meet the quality requirements for in vitro, in vivo pre-clinical validation (proof-of-concept, early safety, potency, efficacy and toxicity studies) and clinical validation.
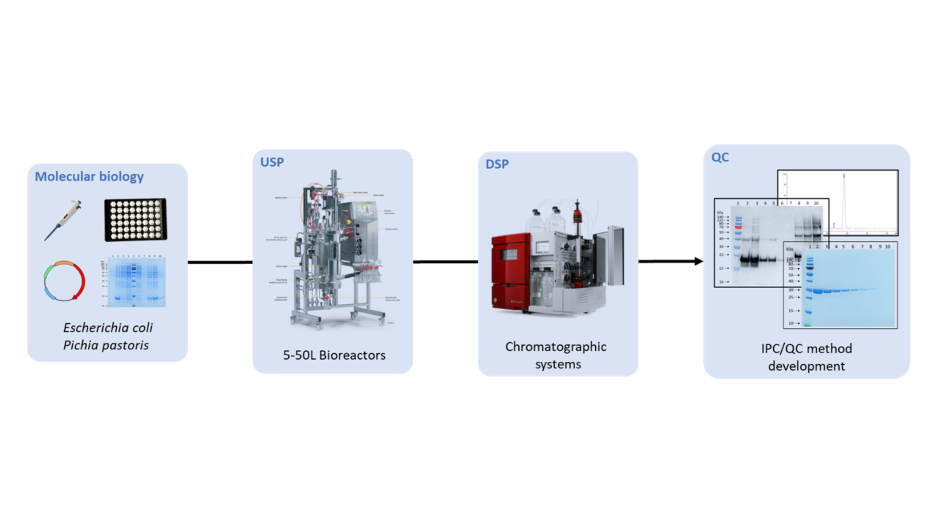 Manufacturing services are based on four technological platforms:
Manufacturing services are based on four technological platforms:

These FAQs are organized by application to guide you to find the best answer possible.

You have access to all the documents related to the transfection reagent.

Search for publications in our Transfection Database with Polyplus transfection reagents

This lexicon will help you to understand the different terms related to Polyplus-transfection®.