in vivo-jetPEI® is a ready-to-use cationic polymer reagent recommended for in vivo transfection of DNA, siRNA, miRNA, shRNA and other oligonucleotide...

Anchiano Therapeutics, formally known as BioCancell Therapeutics, is a pivotal-stage biopharmaceutical company. Their pivotal phase II study with their lead product candidate inodiftagene vixteplasmid (Formally known as BC-819) began in December 2018. This announcement was received with great excitement at Polyplus-transfection® SA: the nucleic acid delivery vector used to target bladder cancer cells with Inodiftagene is none other than in vivo-jetPEI® transfection reagent.
Keywords: Bladder Cancer, Gene therapy, Clinical trial, in vivo transfection reagent, nucleic acid delivery
Anchiano’s lead product was developed based on the discovery of the H19 gene, by Professor Avraham Hochberg from the Hebrew University of Jerusalem. H19 is a controlling element for key malignant cell processes that plays a role in carcinogenesis, tumor progression, drug resistance and metastasis in a spectrum of human cancers. H19 is normally expressed at low levels in most adult somatic tissues, but it is expressed at high levels in a wide variety of malignancies, including in bladder cancer. Inodiftagene vixteplasmid, the company’s lead candidate, is a first-of-its kind gene therapy. Inodiftagene is a targeted recombinant DNA construct that employs H19 gene regulatory sequences in order to drive the expression of an encoded lethal toxin (Diphtheria Toxin A) specifically in malignant cells. Inodiftagene therapy currently addresses the unmet needs in the treatment of BCG-unresponsive non-muscle invasive bladder cancer patients.
Once delivered inside a bladder cancer cell, the H19 regulatory sequence is recognized to drive the expression of the toxin, which inhibits protein synthesis leading to tumor cell death. Engineering the gene construct that would carry inodiftagene within bladder cancer cells to drive their own demise was one of the challenges that Anchiano had to address. Anchiano did so with a safe, non-viral, efficient in vivo nucleic acid delivery method that could be used in early development studies and in clinical trials. This is where Polyplus-transfection’s expertise in nucleic acid delivery fit right in with transfection reagent: in vivo-jetPEI®. How does it work? On a molecular level, in vivo-jetPEI® transfection reagent is able to encapsulate and condense inodiftagene into tiny nanoparticles. These in vivo-jetPEI®-DNA nanoparticles are infused in the bladder of patients and taken up by bladder cells through a common cellular mechanism known as endocytosis. Once inside the cell, inodiftagene is released from in vivo-jetPEI® and is only “activated” in bladder cancer cells that contain H19 transcription factors.
Ten years later, where do we stand? Anchiano Therapeutics has undertaken three clinical trials to date in bladder cancer; successfully completed a phase 1/2 dose-escalation trial and a phase 2 study utilizing inodiftagene as monotherapy. The third trial was a phase 2 trial of inodiftagene administered as induction therapy in combination with BCG. All inodiftagene treatments for the bladder indication, included in vivo-jetPEI® transfection reagent as the non-viral vector for inodiftagene nucleic acid delivery. Polyplus-transfection continues to support cell and gene therapy by providing GMP grade in vivo-jetPEI® for several ongoing clinical trials, and to invest in next-generation nucleic acid delivery methods to support innovation. “Over the years, we have grown together and exchanged a lot scientifically. At Polyplus-transfection, we believe that our successes are entwined and we look forward to the future with great enthusiasm”, states Pascale Belguise, PhD, Business Development Director at Polyplus-transfection.
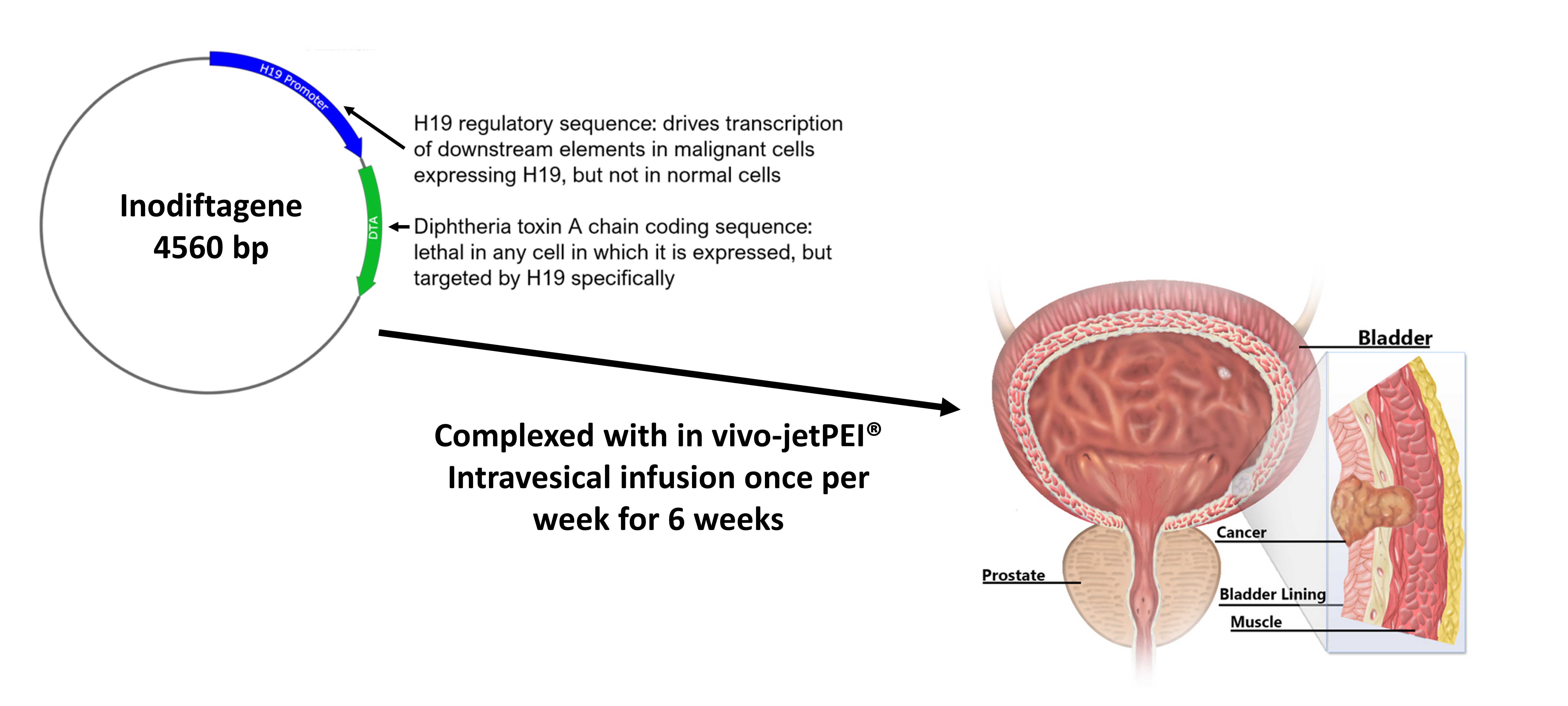
Inodiftagene gene therapy for treatment of early stage bladder cancer. In vivo-jetPEI® enables delivery of plasmid inodiftagene to drive expression of diphtheria toxin A chain only in malignant bladder cells. Intravesical infusion of in vivo-jetPEI®/inodiftagene complexes is conducted once per week over 6 weeks in early stage bladder cancer patients.
5/21/2019