PEIpro® is the leading PEI-based DNA transfection reagent that offers flexibility and scalability for viral vector manufacturing.
Cell and gene-based therapies present a new treatment paradigm that have the potential to address unmet clinical needs. Viral vectors such as adenoviruses, adeno-associated viruses, retroviruses and lentiviruses are effective delivery systems for genetic material used in cell and gene therapies and vaccines. HEK-293 cells and derivatives are commonly used as a workhorse cell line for lentiviral vector (LVV) production for cell and gene therapy applications. Adherent production processes with these cells utilize static flask cultures, and this adherent method is quite easy to develop and perform. However, it also significantly lacks the ability for automation and scalability. Typical bioreactors based either on a rocking motion or stirred tank agitation can provide these features. Therefore, to scale up viral vector production for commercialization, adherent processes should be shifted to a suspension-based process, a significant challenge for the regenerative medicine industry. The use of a suspension adapted HEK-293 cell line and the Ambr® 15 microbioreactor system can facilitate transition from adherent cultures to suspension cultures by enabling fast process optimization with the ability to screen in parallel many parameters in small volumes. As a proof-of-concept study, we established here such a transitional protocol for the cultivation of suspension adapted HEK-293T cells and the production of CD19-CAR lentivirus in small and benchtop scale stirred bioreactors.
Authors: Diana Riethmüller, Dr Alengo Nyamay’antu, Dr Franziska Bollmann.
10.18609/cgti.2021.099
Citation: Cell & Gene Therapy Insights 2021; 7(6), 689–700
Cell and gene-based therapies present a new treatment paradigm that have the potential to address clinical needs that are unmet by current small molecule and biotherapeutic approaches.
Viral vectors such as adenoviruses, adeno-associated viruses and retroviruses are effective delivery systems for genetic material used in cell and gene therapies and vaccines. Lentiviruses are used for example for the transfer of genetic information for novel cellular immunotherapies (gene modified cell therapies), like CAR-T cell therapy. These innovative approaches will be a substantial part of next-generation therapies to cure devastating diseases.
The number of clinical candidates is growing rapidly and commercial scale manufacturing is becoming a reality for these clinical candidates. The processes to manufacture viral vectors for commercialization require a high level of operator expertise and GMP guideline application; yet they are currently mainly based on an R&D approach.
HEK-293 cells and derivatives are commonly used as a workhorse cell line for lentiviral vector (LVV) production for cell and gene therapy applications. Adherent production processes with these cells utilize static flask cultures, like T-flasks, cell factories or cell stacks. This adherent method is quite easy to develop and perform. However, it also significantly lacks the ability for automation and scalability. Typical bioreactors based either on a rocking motion or stirred tank agitation can provide these features. Therefore, to scale up viral vector production for commercialization, adherent processes should be shifted to a suspension-based process, a significant challenge for the regenerative medicine industry. Suspension-based lentivirus production could either be performed using microcarriers for culturing adherent cell lines or by using a suspension adapted cell line. The use of a suspension adapted HEK-293 cell line and the Ambr® 15 microbioreactor system can facilitate transition from adherent cultures to suspension cultures by enabling fast process optimization with the ability to screen in parallel many parameters in small volumes. As a proof-of-concept study, we established here such a transitional protocol for the cultivation of suspension adapted HEK-293T cells and the production of CD19-CAR lentivirus in small and benchtop scale stirred bioreactors.
Ambr® 15 from Sartorius is an automated micro-scale bioreactor system that enables a fast screening of process parameters such as pH, DO, temperature, stirring rate in less time, with reduced reagents/media use and labor costs. This is a financial benefit for CDMOs and start-up companies. Parallel processing, automation capability and consistency provided by Ambr® 15 system, enables rapid, high throughput process improvement and optimization, including Design of Experiment (DoE) studies. It releases efforts for time spent in data analysis, thanks to its integration to the DoE software MODDE®.
The “big brother” of Ambr® 15, the Ambr® 250 Modular, is a bioreactor system with working volumes going from 100–250 mL and a system for scale-down model for larger stirred bioreactor systems. Similarly, to the Ambr® 15, the Ambr® 250 Modular facilitates upstream process development with reduced effort due to its parallel cultivation capacity and the possibility for “hands-off” workflow automation. The Ambr® 15 and Ambr® 250 have been shown to be valuable scale-down model systems. Although some effort is needed to characterize them and also novel scale-down model criteria might need to be established, due to the high throughput screening capabilities of both instruments, this characterization can be efficiently performed. Some process characteristics cannot be mimicked with the Ambr® 15 and the Ambr® 250 Modular like any continuous manipulation, e.g. perfusion or feeding cultivation strategies. Figure 1 highlights the beforementioned capabilities of the Ambr® bioreactor systems. Due to their scalability to larger stirred bioreactor systems, they are the ideal tools for process development.
We first focused on optimization of cell culture conditions. We used the Ambr® 15 microbioreactor system to screen for highest viable cell count and highest lentivirus titer while varying key parameters such as stirring speed and pH value. The factors have mainly been selected based on previous findings. Some factors, e.g. the seeding cell density and percentage of DO have been optimized prior to the study presented here. Secondly, we optimized the transient transfection protocol with PEIpro® transfection reagent to reach high and robust recombinant lentivirus titers. Optimization of transient transfection requires taking into account several parameters, including selection of synthetic culture media, DNA amount, ratio of DNA / PEIpro® transfection reagent and ratio of plasmids. Thirdly, we wanted to assess scalability of optimal conditions by producing lentivirus in larger volumes in the Ambr® 250 Modular. The methods used for lentivirus production and quantification were mainly based on those described by Labisch et al.. To get meaningful results with reduced sample number, we performed a DoE study to identify optimal culture and transfection conditions by using the MODDE® software for experimental planning and analysis of results.
To quickly identify optimal culture conditions to produce lentiviral vector, parallel analysis in an automated fashion was performed in the small-scale bioreactor Ambr® 15 system combined with DoE principles. We optimized the stirring speed and the cell culture pH within a defined range, as shown in Table 1.
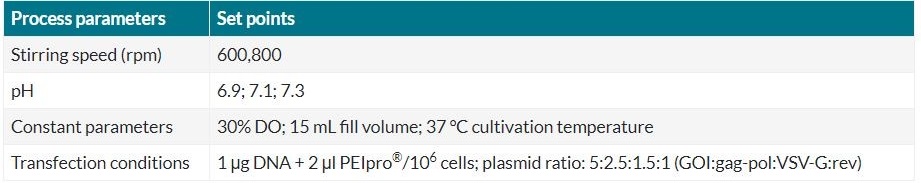
Table 1 Cultivation and transfection conditions of experiment 1 (Ambr® 15).
These two factors were evaluated as to their contribution to the lentivirus titer yield (Figure 2). According to Figure 2A, cells generally grew better in the Ambr® 15 than in the control shake flask culture. However, large differences in the fold expansion can also be observed between the different conditions tested in the Ambr® 15. The viability of the cells cultured at pH 7.3 drops significantly at day 2 (not shown) which explains the reduced lentivirus titer at this pH. Overall, transient transfection with PEIpro® did not impact cell viability (data not shown). Generally, it was observed that higher pH values and stirring speeds yielded improved cell growth (Figure 2A), but these factors negatively correlate to LV particle titer as seen in the DoE model (Figure 2B). This may imply that a 2-step approach could be beneficial with a shift of the main process parameters when transitioning from the growth phase to the production phase at the time of the plasmid transfection.
We also identified that the lentivirus titer is higher in the Ambr® 15 vessels than in the positive control shake flask. According to Figure 2A we could clearly identify optimal culture conditions for lentivirus production in the Ambr® 15 microbioreactor. A stirring speed of 600 rpm and a pH between 6.9 and 7.1 yielded the highest lentivirus titer (8.8 x 109 – 9.8 x 109 VP/mL). This trend was confirmed with the DoE model. According to the response contour plot a clear trend of an increasing lentiviral particle titer could be observed with a decreasing stirring speed and a peak lentiviral titer was observed at pH culture values between 7.0 and 7.1. Furthermore, it can be concluded that the culture pH and the stirring speed are critical process parameters that have a significant effect on the viral vector production.

To optimize the transient transfection process, we used PEIpro® transfection reagent in the Ambr® 15 microbioreactor system. PEIpro® benefits from extensive research development that make this unique PEI-based transfection reagent optimal for lentivirus production in adherent and suspension systems system.. This is in part due to its unique ability to efficiently condense several plasmid DNA and deliver them into HEK-293 cells for production of full lentivirus particles. We optimized four parameters that could have an impact on the success of transient transfection and therefore on virus production: the viral production medium, DNA amount, ratio of DNA/transfection reagent and ratio of plasmids.
Through a DoE approach we were able to screen all these parameters in one cultivation run, thanks to the screening capabilities of the Ambr® 15 microbioreactor system. Recapitulated in Table 2 are the ranges tested for each parameter, based on previous experience and manufacturers protocols. A D-optimal design with triplicate center points was chosen, leading to 23 different conditions / vessels.
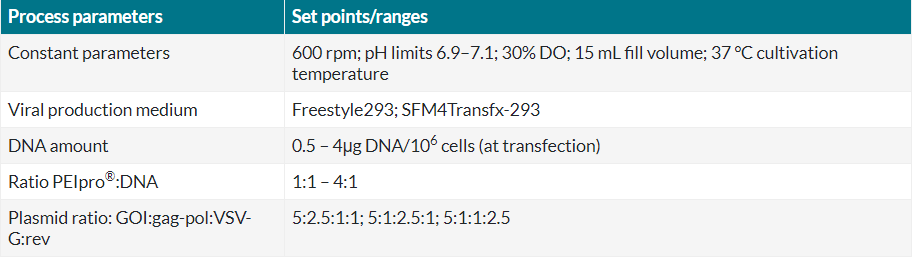
Table 2 Cultivation and transfection conditions of experiment 2 in Ambr® 15.
According to Figure 3A, the LV particle titer is higher in some of the Ambr® 15 vessels than in the shake flask positive control. Depending on the conditions used for transfection, extreme differences in titers between the culture vessels can be observed, indicating that the factors analyzed indeed have a significant effect on lentivirus productivity. At optimal conditions, a viral titer of 2.1 x 1011 VP/mL and a specific productivity, meaning the particle titer per cells at the time of transfection, of 1.3 x 105 VP/cell was obtained which was higher than the titer obtained in the reference shake flask (1.4 x 1011 VP/mL and 5.9 x 104 VP/cell).
The infectious viral titer of the best condition in the Ambr® 15 seems to be equal to the positive control shake flask, however, when comparing the specific productivity of the lentivirus, meaning the infectious titer per cells at the time of transfection, this value is significantly higher for the Ambr® 15 vessel (20.13 for the Ambr® 15 vs. 13.66 TU/cell for the reference shake flask).
Furthermore, when comparing the highest viral particle titer obtained in this lentivirus production run with the highest one from the first experiment, we see another twentyfold increase in viral particles through this optimization.
After obtaining the LV particle titer for all culture vessels, we analyzed the DoE model of this screening experiment with the MODDE® software. Our results lead to a good modeling of the DoE for the optimization process. Furthermore, when we plotted the results in a response contour plot, we could clearly see an optimal spot of the lentivirus particle titer when the ratio of PEIpro® to DNA was high and the DNA amount per 106 cells was low. We were able to identify several factors that have a significant effect on the lentivirus titer during the transfection process. For example, the ratio of the amount of PEIpro® to DNA, the usage of Freestyle 293 medium and the plasmid ratio of 5:2.5:1:1 positively correlate to LV particle titer. However, the amount of DNA, the usage of SFM4Transfx 293 medium and a plasmid ratio of 5:1:2.5:1 negatively correlate with the lentivirus titer. The plasmid encoding the gene of interest (GOI) was always present in excess due to the larger size of this plasmid with the GOI to improve virus particle production capacity. Reducing the VSV-G amounts while increasing gag-pol amounts provided a good balance between proteins needed for virus replication and proteins involved in the LV enveloped particles formation.
Therefore, with only one production run we were able to identify optimal set points of key factors which led to a twentyfold increase in lentivirus titer compared to the initial protocol used in experiment 1.
According to the optimizer function of the DoE software MODDE® our new optimal conditions for the transfection step during lentivirus production are:
DNA amount: 0.5 µg/106 cells; ratio PEIpro®:DNA: 4:1; viral production medium: Freestyle 293; plasmid ratio: 5:2.5:1:1 (GOI:gag-pol:VSV-G:rev)
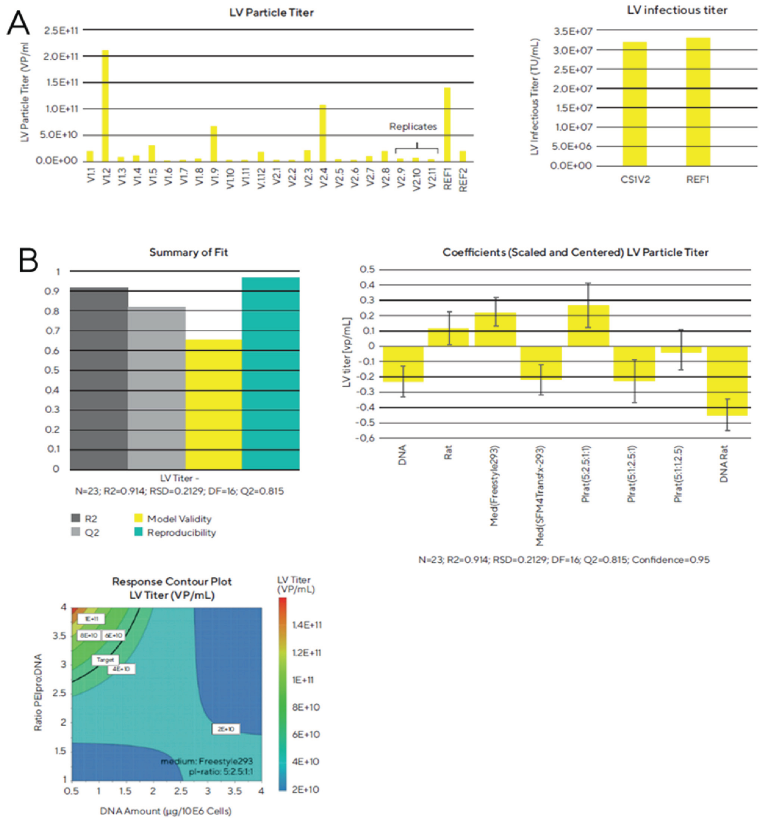
Figure 3 (A) Lentiviral particle and infectious titer obtained in a screening experiment to optimize the transfection process with the Ambr®️ 15. (B) Analysis of the DoE model of the transfection conditions screening experiment.
After having optimized the transient transfection process step and cultivation parameters for lentivirus production, we tested the feasibility of upscaling the optimized process to the Ambr® 250 Modular. We furthermore aimed to optimize the gas flow rate and stir direction which is enabled in this bioreactor system with automated processing. These parameters could potentially have a significant impact in the viral titer due to its sensitivity to externally applied forces (i.e., shear forces) and the dependence of the viral titer on the cell viability.
The culture parameter set points and transfection conditions are listed in Table 3. Due to the limited scalability of the Ambr® 15 system, the optimal stirring speed for the production process in the Ambr® 250 Modular was identified by running a separate experiment which included testing of four different stir speeds in the Ambr® 250 Modular.
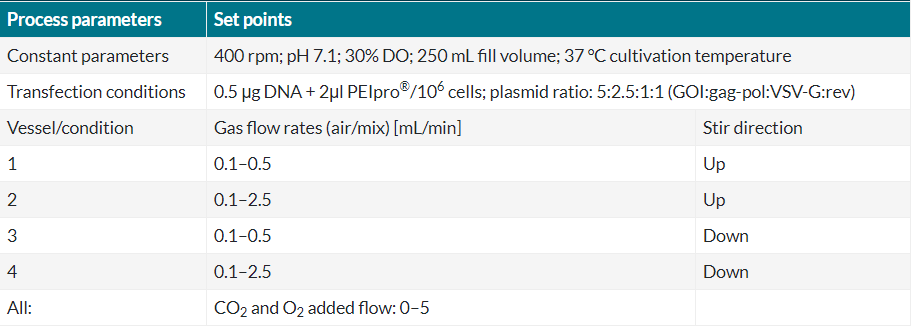
Table 3 Gas flow rates and stir directions of experiment 3 (Ambr® 250 Modular).
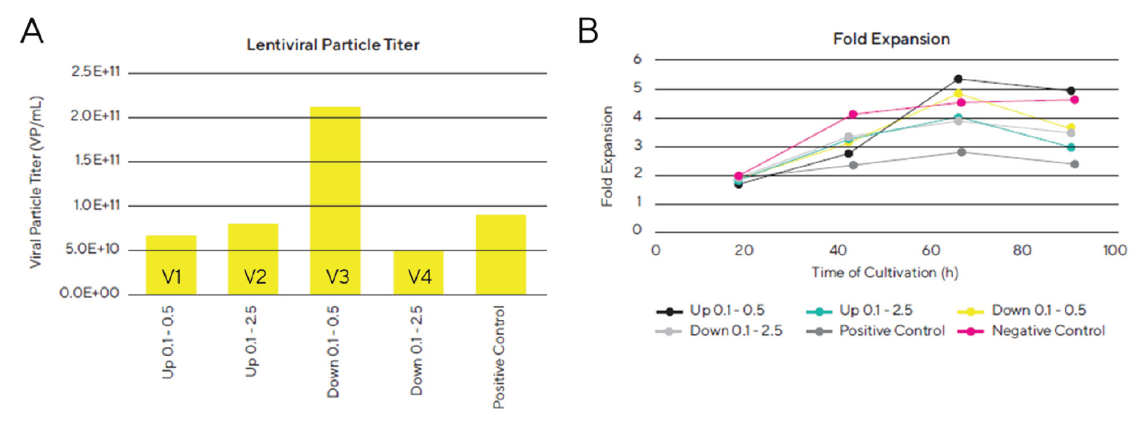
According to the results shown in Figure 4, the lentiviral particle titer was significantly higher in vessel/ condition 3 of the bioreactor system which corresponds to a maximum gas flow rate of 0.5 mL/min and a down stirring direction of the impellers. In this vessel, a viral titer of 2.1 x 1011 VP/mL and a specific productivity of 1.3 x 105 VP/cell was obtained which was higher than the titer obtained in the reference shake flask (8.9 x 1010 VP/mL and 3.7 x 104 VP/cell).
The highest viral titer was obtained with the lowest gas flow rate and we could prove that the gas flow rate and the stir direction have a significant effect on lentiviral particle titer, indicating a negative impact of high gas flow rates on lentiviral titer. Varying flow rates and stir directions also led to differences in fold expansion of the cells during lentivirus production. Even though the viral particle titer was very high in vessel 3, we also observed a good growth rate of the cells in this bioreactor. In general, the cells cultured in vessel 1 and 3 showed a better growth profile than vessel 2 and 4, indicating that the gas flow rate has a major impact on cell growth and viability. In this experiment we were able to show that the optimized lentiviral vector production protocol is scalable to larger bioreactor volumes, and that the gas flow rate had a significant impact on lentiviral titer. With the most optimal condition, we were able to obtain a lentiviral particle titer of 2.1 x 1011 VP/mL and a specific productivity of 1.3 x 105 VP/cell in Ambr® 250 Modular which was consistent with the optimized LV production in Ambr® 15 (2.1 x 1011 VP/mL and 1.3 x 105 VP/cell). These results confirm that production of lentiviral vectors can directly be scalable from the Ambr® 15 microbioreactor system to a larger stirred bioreactor system without loss in yield.
This study demonstrates that the Ambr® 15 microbioreactor system in combination with the DoE software MODDE® enables a systematic investigation of critical process parameters and rapid, high throughput process optimization in a reduced time. Optimization of cultivation parameters and of the transfection process is critical to substantially improve lentiviral titer.
The results prove that the transition from shake flask to a scalable stirred bioreactor system can be facilitated and lead to further improved lentiviral titers. Optimization can directly be ensured with the right set of tools: the automated Ambr® 15 microbioreactor system combined with a scalable transfection process with PEIpro® transfection reagent. Scale-up of the process is simplified by relying on the Ambr® 250 Modular. The Ambr® 15 correlated well with the Ambr® 250 Modular results and provides good basis for further scale-up to larger stirred bioreactors as shown previously for a mAb process.
The outcome of such a study is designed to help manufacturers gain important process knowledge on the parameters that need to be controlled to set up a robust and predictable lentivirus production process that supports scaling to much larger scales (up to 2k L) for GMP manufacturing at commercial scale.
Third generation lentivirus was produced by transient transfection of suspension HEK-293T/17 SF cells (ATCC #ACS-4500) in a stirred bioreactor (either Ambr® 15, Ambr® 250 Modular).
The cells had been passaged at least twice before starting the lentivirus production.
Freestyle 293 media (Thermo Fisher Scientific) was filled into the respective bioreactors on day 0 and process parameter control was initiated. Later, on day 0, the cells were seeded into the bioreactor at a final VCD of 1 x 106 cells/mL. A lentivirus production in a 125 mL shake flask was prepared equally to the bioreactor (positive control).
After 24 h, transfection of a CD19-CAR encoding transfer plasmid and three lentiviral helper plasmids (Aldevron) are performed using PEIpro® DNA transfection reagent (Polyplus Transfection). A defined amount of DNA (sum of all four plasmids) per 1 x 106 cells is diluted in Freestyle 293 medium at a certain plasmid ratio (the volume is 1:20 of the final culture volume). In a separate reaction tube a defined volume of PEIpro® per 1 x 106 cells is diluted in Freestyle 293 medium (the volume is 1:20 of the final culture volume). Diluted PEIpro® is added to the diluted DNA, gently mixed and incubated for 15 min at room temperature. The mixture is added dropwise to the cells. A negative control without using a transfection reagent is prepared and treated equally (cells are cultured in a 125 mL shake flask).
On the next day, i.e. 18 h after transfection, anti-clumping reagent (1:500 (v/v), Thermo Fisher Scientific) and 10 mM sodium butyrate (Sigma) are added.
LV was harvested 72 h post transfection. Before harvesting, the virus suspension was treated with 10 U/mL DENARASE® (c-Lecta) for 1 h for digestion of nucleic acids.
As a primary readout on virus concentration, we performed a p24-ELISA, that measures lentivirus-associated p24 protein, to determine the total viral particle titer. The assay was performed according to the manufacturer´s protocol (Cell Biolabs). The assay’s accuracy was determined to be below 8 % CV.
Due to the nature of typical infectious titer assays, being very laborious and giving low sample throughput, we decided to primarily run a particle titration assay (p24-ELISA) and only determine the infectious titer of selected samples based on the viral particle titer results. Still, viral particle titers allow for observation of overall effects of factors on the lentivirus production process.
A flow cytometry-based assay was performed to determine the infectious lentiviral titer by transducing adherent HEK-293T cells with the lentiviral supernatants.
The HEK-293T/17 SF cell density and viability were measured with a Cedex HiRes instrument (Roche).