pPLUS® AAV-RC range is a set of ready-to-use RepCap plasmids for AAV production of various serotypes through triple transfection. Optimized for use w...

|
|
|
|
|
|
Adeno-Associated Viruses (AAVs) are small viruses made of a single stranded DNA genome (4.7 kb) encapsidated in a viral capsid made by assembly of three proteins (VP1, VP2 and VP3). Wild-type AAVs require co-infection with a helper virus, such as adenovirus (Ad), herpes simplex virus, or vaccinia virus, to allow productive infection and replication. Upon active infection, the AAV genome persists in the host cell nucleus in an episomal form with sustained RNA expression and can also integrate the host DNA into chromosome 19.
Recombinant adeno-associated viruses (rAAV) are used as a DNA delivery vector for human gene therapy, relying on the ability of AAVs to infect human cells while being non-pathogenic. rAAVs are produced and administered without any helper virus, which lowers the risk of genome integration and confers these vectors with an interesting safety profile. The various AAV serotypes enable specificity to deliver the therapeutic Gene of Interest (GOI) into the targeted organs.
The demand for rAAV is growing rapidly as clinical pipelines expand and new gene therapy approvals are granted. However, the manufacturing process for AAV remains complex and challenging when it comes to scaling-up and achieving high yields. There is a strong need to find the optimal conditions contributing to large-scale AAV production process intensification.
Currently, the most common approach to manufacture rAAVs at large scale is the use of triple transfection in suspension cultured HEK293 cells followed by a purification process.
Production of rAAVs commonly involves chemical-based transfection of plasmid DNA delivering genetic material into HEK293 cells. Cell machinery is rerouted to enable the production of viral proteins required to build novel rAAV particles that carry the therapeutic GOI.
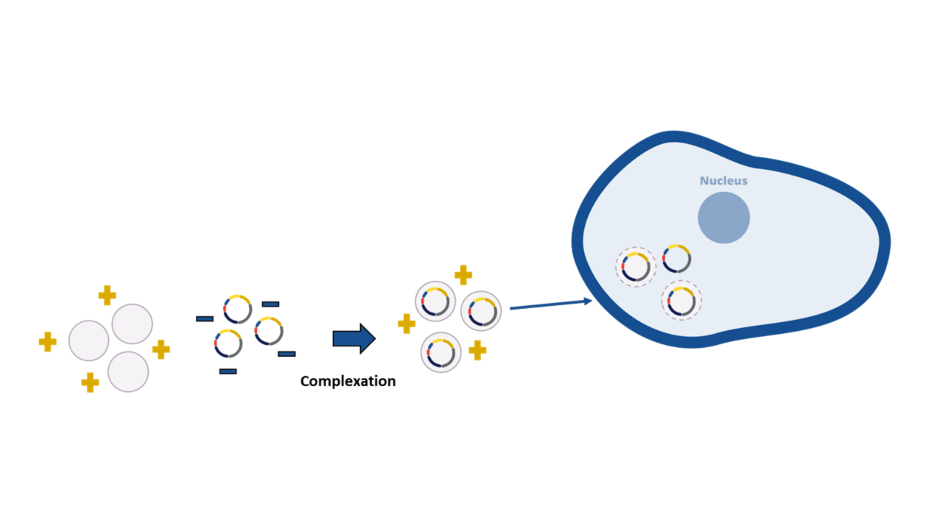
| Find out more about polymer-based transfection reagents: FectoVIR® AAV and PEIpro® |
To produce rAAVs, the most established method requires a co-transfection of three plasmids, one transgene encoding for the therapeutic GOI and two packaging plasmids, each one bringing specific functionalities as described below
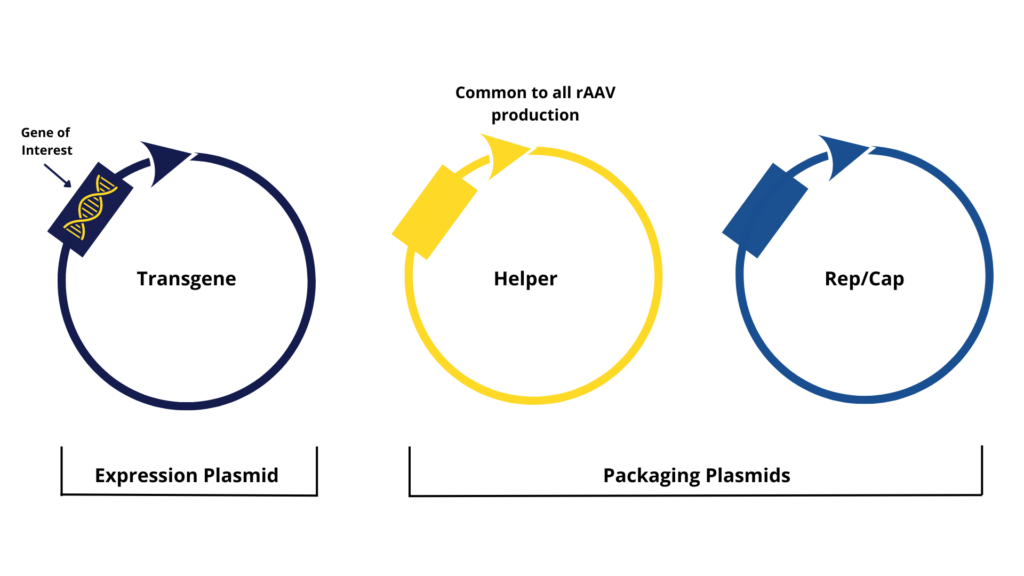
As wild-type AAVs require co-infection with a helper virus to allow productive infection and replication, a helper plasmid ‒ containing necessary genes to support rAAV replication and encapsidation ‒ is transfected together with the Rep/Cap plasmid. The combination of both plasmids is necessary for successful “packaging” of the GOI into viral particles.
The helper plasmid typically contains critical adenoviral genes required for rAAV replication (E1A/B, E2A, E4, and VA RNAs). However, other viruses are known to be helper viruses of wild-type AAVs such as Herpes Simplex Virus (HSV) or Human Bocavirus (HboV).
| While traditional helper plasmids contain helper genes from adenoviruses, the pPLUS® AAV-Helper also contains additional helper sequences sourced from HSV and HboV which are both helper viruses of wild-type AAVs as reported in literature. This helps to improve efficiency of viral particle packaging, ultimately improving rAAV quality and potency over traditional helper plasmids.
Critical genetic sequences encoding for helper proteins have been isolated and selected through a large screening of different plasmid constructs. This resulted in the addition of two new helper genes, namely UL12 from HSV-1 and NS2 from HBoV-1, to the common E2A, E4, and VA-RNA genes from adenovirus. |
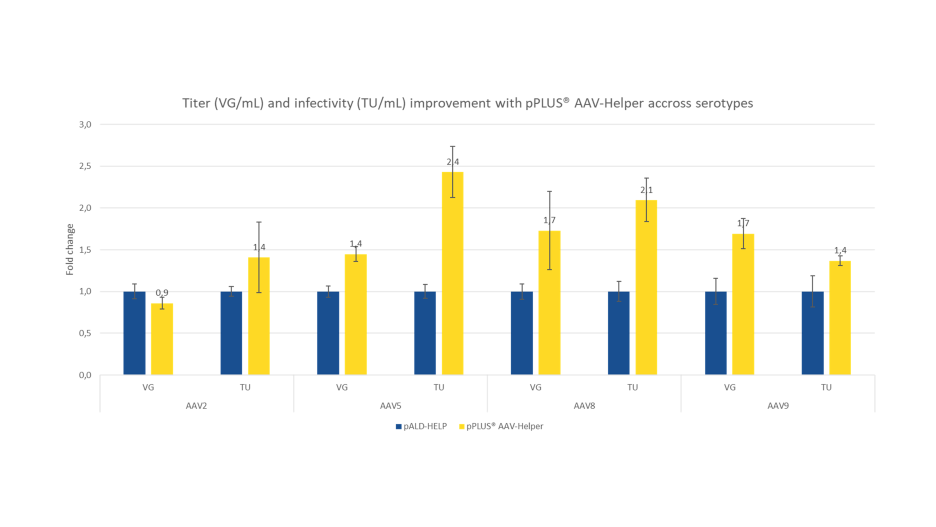
The figure below demonstrates that the pPLUS® AAV-Helper improves rAAV infectivity as measured in Transducing Units (TU) titer, suggesting a higher quality profile of the produced rAAVs. AAV2, AAV5, AAV8, and AAV9 were produced in HEK293T cells adapted to suspension in F17 medium and transfected with FectoVIR®-AAV. Transduction assay was performed on HT-1080 cells. Data normalized to pALD-HELP (Aldevron) used as control.
| When testing new raw materials, such as transfection reagents or plasmid DNA, standard recommendations are often used as a starting point. However, it is critical for AAV yield enhancement to make sure that the process is optimized by taking into consideration the relevant working conditions (ie. cell line, medium, pDNA, etc.).
Process optimization can be achieved through design of experiments (DOE) methodology. In the case of introducing new plasmids for transfection, using a mixture design approach is recommended to reveal what the optimal plasmid ratio is. |
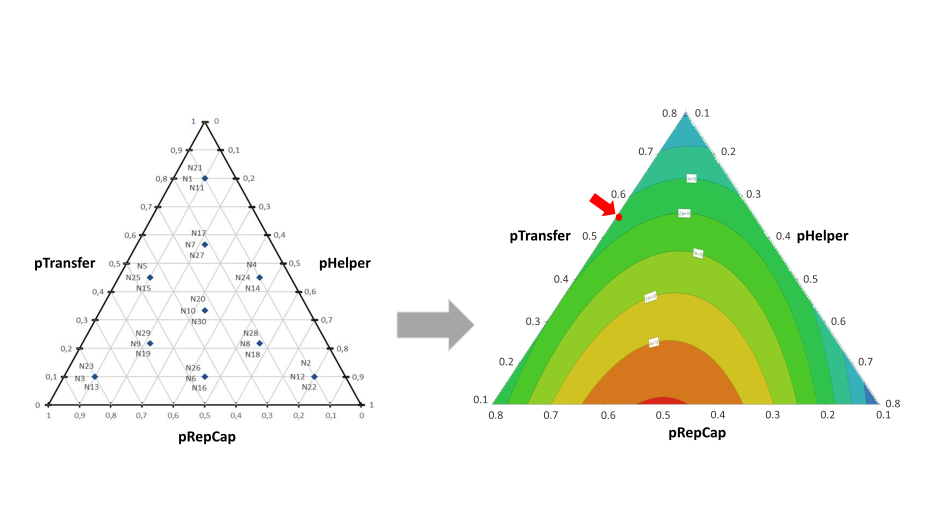
During the development and validation of the pPLUS® AAV-Helper, the experimental set-up to produce AAV9 in VPC2.0 cells was found to be challenging when using standard plasmid ratios. In the first experiments of AAV9 production with this system, the obtained VG titers were insufficient. Starting from there, the hypothesis that the plasmid ratio used was not optimal for this condition was considered and a first DOE experiment using mixture design was performed to determine whether performance could be further enhanced by fine-tuning plasmid ratios.
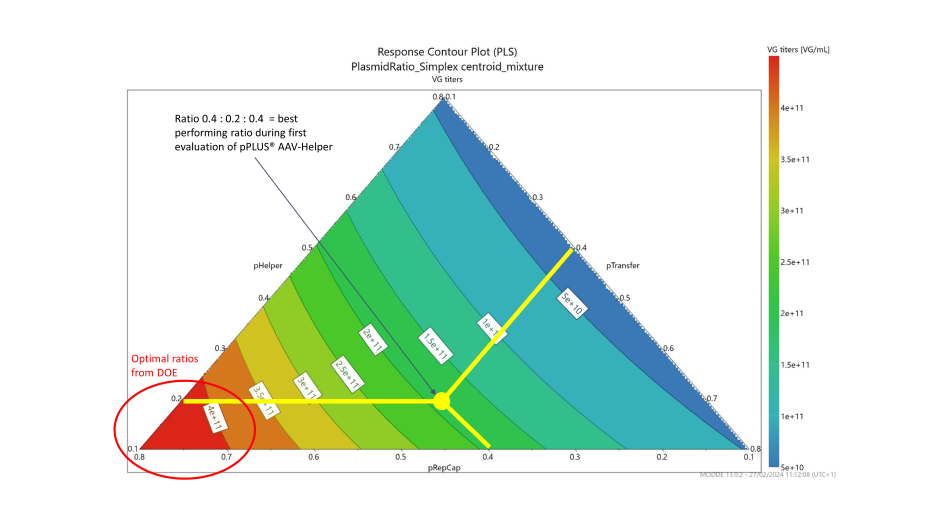
For this DOE, MODDE® Pro Software from Sartorius was used to establish the experiment design. Ten different plasmid ratios were evaluated using a mixture design approach as shown in the graph below on the left.
Both VG titers and VP titers were measured for each data point. The DOE results were analyzed using MODDE® Pro. The generated contour plot revealed that the optimal ratios were close to 4.5:4.5:1 (Helper:RepCap:Transgene) as shown in the below graph on the right. The red arrow positioned on the contour plot shows the initial plasmid ratio used in the development.
| As a second step, the DOE was refined by focusing the experiment design upon a limited set of conditions identified from the first contour plot.
Nine different plasmid ratios were evaluated using a D-Optimal mixture design. Both VG titers and VP titers were measured for each data point- and the DOE results were analyzed using MODDE® Pro. |
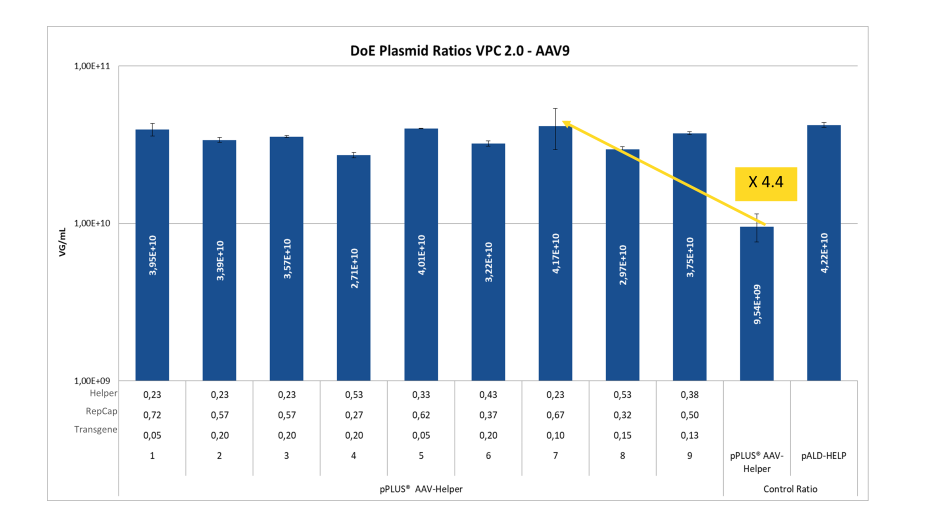
As shown in the above graph, the optimal ratio in terms of VG titers in this experiment was found to be [2.3 : 6.7 : 1]. By using this ratio, the VG titer was able to be increased by a factor of 4.4 (condition 7) compared to the initial ratio used during the development of pPLUS® AAV-Helper (mentioned here as “control ratio”).
The results also show that the first DOE identified the optimal area for results, which in the confirmation run was shown to be relatively broad, giving a range of optimal plasmid ratios with consistent results.
This approach confirmed that the performance of a new optimized plasmid can be further enhanced by assessing plasmid ratios through DOE to define the optimal ratio for each production process.
Further improvements may be achieved by extending this DOE to other factors (DNA quantity, FectoVIR®-AAV:DNA ratio, etc…)
In this case study, the pPLUS® AAV-Helper was evaluated by ABL Europe, a GMP CMO, completely dedicated to the provision of viral vector GMP contract manufacturing services.
Experiments were done using VPC2.0 cells (Thermofisher) grown in Viral Production Medium (ThermoFisher). VPC2.0 cells were transfected using FectoVIR-AAV with pALD-AAV2 (Aldevron), pPLUS® AAV-Helper, and the third-party’s undisclosed pRepcap plasmid .
In the first experiment, pPLUS® AAV-Helper performance was assessed against the pHelper plasmid from Aldevron, pALD-HELP, at three different standard plasmid ratios (expressed as mass ratios Transgene:Helper:RepCap):
| In this first experiment, similar genomic titers (measured by qPCR on ITRs) were obtained with the two helper plasmids, whichever ratio was used.
However, full/empty ratios, measured through the ratio between VG titers and VP titers, were significantly increased when using pPLUS® AAV-Helper. |
From there, the decision was made to evaluate whether VG titers obtained in this experiment with pPLUS® AAV-Helper could be further enhanced through plasmid ratio optimization.
| Through a mixture design approach, 10 different plasmid ratios were evaluated. Both VG titers and VP titers were measured for each data point.
The DOE results were analyzed using MODDE® software. |
The contour plot obtained from this DOE highlighted an optimal zone for VG titers corresponding to a ratio 1:1:8, highlighting a need for more RepCap plasmid than transgene and GOI in those testing conditions. When using this ratio, a titer of 4.54E+11 VG/mL was obtained.
The ratio tested in the initial evaluation (2:1:2) was positioned on the contour plot and the theoretical titer was back-calculated based on the data model generated by MODDE® Pro. By optimizing the ratio, a theoretical 2.5-fold improvement could be obtained. While the optimal ratio for VG titers was close to 1:1:8 in this DOE, it was interesting to verify whether this would also apply to full / empty ratio. Based on VG and VP titers, the contour plot for full / empty ratio was also generated from MODDE® Software.
| Interestingly, the best ratio for VG titer does not give the best full / empty ratio, and the trends are in the opposite vectors confirming that there is a tradeoff relationship between VG titer and full / empty ratio.
This means that finding an appropriate compromise between the two parameters may be the right option, as shown below. |
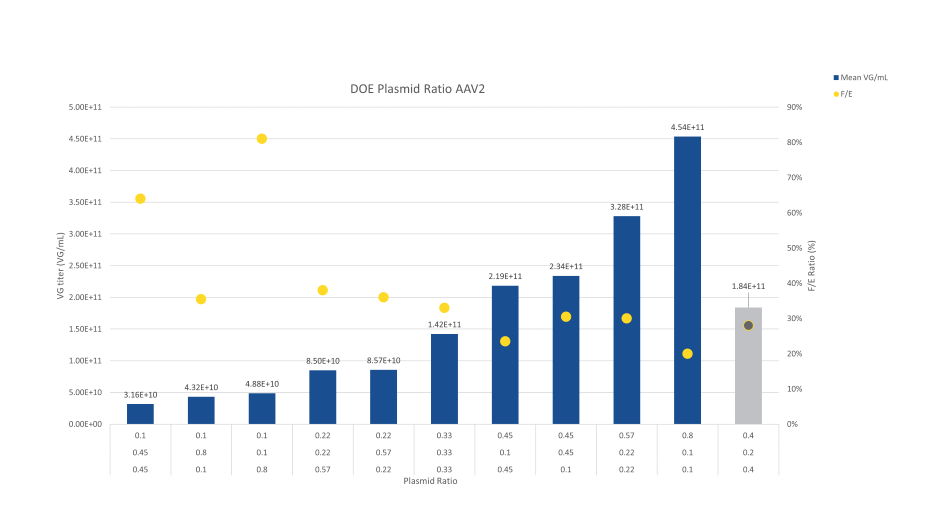
Combining the obtained results for VG titers and Full / Empty ratio shows the tradeoff relationship between the two analytical parameters. Performing the DOE enabled a nice increase in the VG titer compared to the initial ratio tested in the first evaluation (grey bar in the graph).
Condition 10 shows the best improvement in the VG titer (2.5-fold improvement compared to the initial ratio tested) but led to a slight decrease in the Full /Empty ratio, while condition 9 led to a lower increase in the VG titers (1.8-fold improvement) but maintaining the Full / Empty ratio at the same level.
This highlights the importance of monitoring various analytical parameters when optimizing a process, and the need to find a compromise between VG titers and Full / Empty ratio when choosing optimized conditions for production.
| According to MODDE statistical analysis, the best ratio for a compromise between VG titers and % Full in this experimental set-up is: 0.67 : 0.23 : 0.1 (RepCap:Helper:Transgene) as illustrated in the opposite contour plot. |
The pPLUS® AAV-Helper is a novel helper plasmid designed to improve efficiency of viral particle packaging, ultimately resulting in higher rAAV quality and infectivity. When evaluating this new helper plasmid, it was shown that optimizing plasmid ratios is critical to increase the productivity of the process. Optimal plasmid ratios may also vary depending on the analytical parameters evaluated (e.g. VG titers, full / empty ratio, partially filled capsids, etc.). It may be needed to find the best compromise between the different analytical results obtained while taking into consideration that there are often tradeoff relationships between some of those parameters, such as VG titers and full / empty ratio, as shown in the DOE results with AAV2.
The data generated so far with this plasmid also showed that optimal plasmid ratios vary depending on the production conditions (cell line, serotype, GOI, etc.). Therefore, it is highly recommended to use a mixture design approach to discover the best working ratio to fully benefit from the optimization of novel plasmids or when switching the GOI.