in vivo-jetPEI® is a ready-to-use cationic polymer reagent recommended for in vivo transfection of DNA, siRNA, miRNA, shRNA and other oligonucleotide...

Transgene expression in eucaryotic cells is one major application of genetic engineering. Control of transgene expression is dependent on several biological tuning, the first to occur being the transcriptional regulation. When designing an expression vector, one must ask himself about the many variables to be controlled:
– What is the appropriate level of expression required to achieve detection or optimal effect while limiting bias or toxicity?
– Do I need a quick or a late expression? A transient or a sustained one?
– What promoter will be active in my cell lines, tissues or organism?
– Am I limited with the size of the promoter or its CpG content?
Eventually, after having read a couple of papers (sometimes quite ancient ones) or discussed the matter with your good friend who worked the cells two decades ago, you may end up wild-guessing what promoter to use in your construct, and hoping it would do the job. This is a risky way to make a choice, especially if your DNA vectors are complex ones. Nobody likes to realize, 6 months later, that the wrong promoter was picked up, bringing back the project to the starting point.
Deep down, you know: a guess is not a test!
At e-Zyvec we believe your work deserve the best chances to succeed. This starts by accompanying you to answer these crucial questions about promoters, so you can make some informed choice for your project with eucaryotic cell.
How can we do this? By conducting accurate tests of course.
We have devised a series of simple vectors expressing a fluorescent reporter (eGFP) and only differing by the nature of their promoter. We call them PromeZ vectors. Many commonly used promoters are already included in this series (figure 1). All these promoters are also available on our D-Zyvec vector drawing tool, so you can re-use them later in more sophisticated designs.
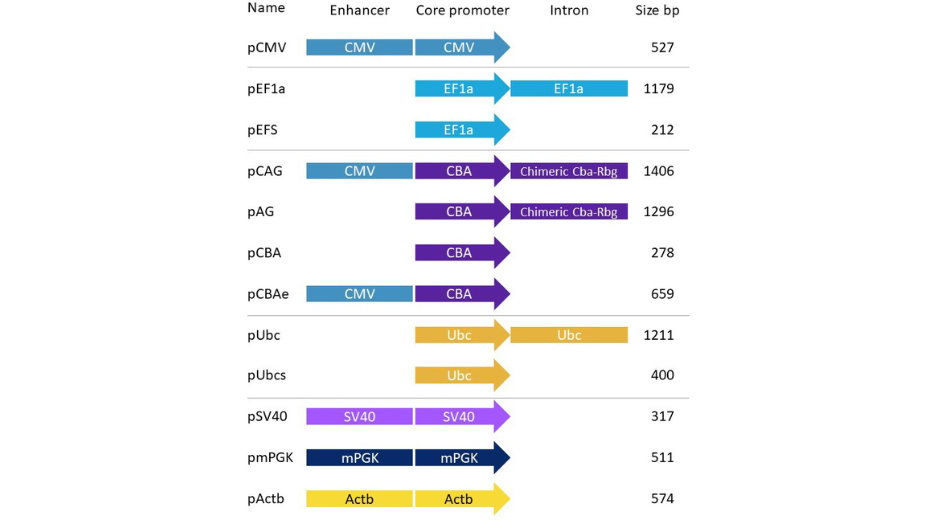
Figure 1
You can buy our PromeZ vectors as modular kits containing the promoters you are interested in, as many as you like. We can also include your promoter(s) of interest, amplified from genomic DNA or obtained through DNA synthesis. This way you can properly test each promoter in your cells and in your own culture conditions and let us know what will work best in your future constructs.
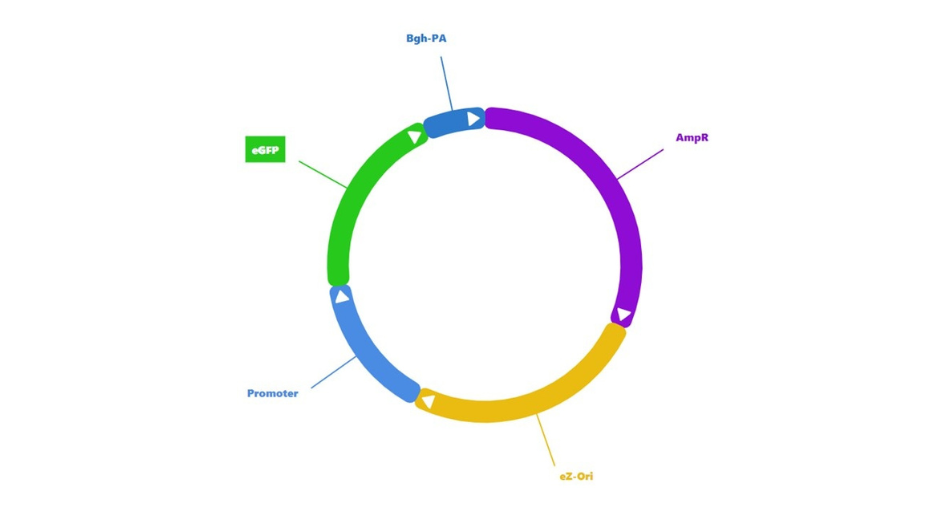
Figure 2
You may not wish to carry out these preliminary studies by yourselves for various good reasons. Not to worry, Promez can be performed by our team. We can arrange to cultivate your eucaryotic cell lines of interest and to transfect them in optimized conditions. Promoter activity is measured using microplate arrays and fluorescence reading. Most of the parameters of the experiment are customizable, such as the cell density, the cell stimulation or the time(s) of measurement.
For example, the diagram below is presenting the results obtained with PromeZ vectors transfected in 104 HEK-293-T cells and analyzed 48hours post-transfection.
Now, looking at these results, may you please ask yourself:
Would you have guessed which of these promoters were the strongest?
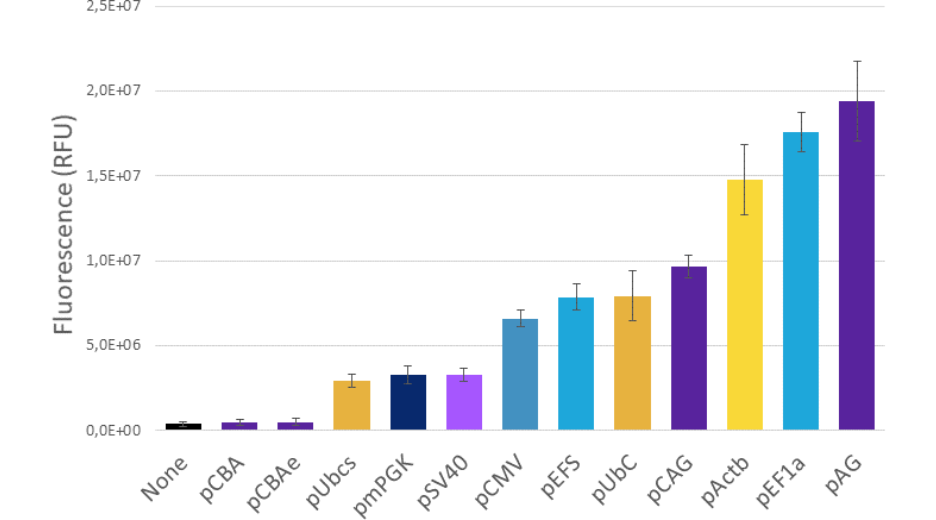
Figure 3